No products in the cart.
Return To ShopRadiometric Dating: Doesn’t it Show that the Earth is 4.5 Billion Years Old?
Secular scientists date the Earth to about 4.5 billion years old by using selected radiometric dating results. Ultimately, what they call “deep time” serves as the very foundation of evolution theory. High school biology books openly acknowledge this necessary connection:
Evolution takes a long time. If life has evolved, then Earth must be very old. Geologists now use radioactivity to establish the age of certain rocks and fossils. This kind of data could have shown that the Earth is young. If that had happened, Darwin’s ideas would have been refuted and abandoned. Instead, radioactive dating indicates that Earth is about 4.5 billion years old—plenty of time for evolution and natural selection to take place.[i]
But as we show here, geologists do not use radioactivity to establish the age of certain rocks. They instead use selected radioactivity results to confirm what they need to see. As discussed in previous chapters, this viewpoint, being secular, contradicts God’s stated Word in Genesis and even the Ten Commandments, where He wrote with His own hand that He created the heavens, Earth, sea, and all that is in them in six days (Exodus 20:11).
Belief in deep time rests upon evolution’s required time. That’s sure putting a lot of faith in something that can’t be tested through direct observation. After all, plenty of assumptions go into the calculations, as we’ll discuss in this chapter.
Keep in mind that while this chapter reviews the technical details behind radiometric dating, only two very basic but completely catastrophic “fatal flaws” undermine radiometric dating.
The first fatal flaw is that it relies upon untestable assumptions. The entire practice of radiometric dating stands or falls on the veracity of four untestable assumptions. The assumptions are untestable because we cannot go back millions of years to verify the findings done today in a laboratory, and we cannot go back in time to test the original conditions in which the rocks were formed. If these assumptions that underlie radiometric dating are not true, then the entire theory falls flat, like a chair without its four legs.
The second fatal flaw clearly reveals that at least one of those assumptions must actually be wrong because radiometric dating fails to correctly date rocks of known ages. For example, in the case of Mount St. Helens, we watched rocks being formed in the 1980s, but when sent to a laboratory 10 years later for dating, the 10-year-old rocks returned ages of hundreds of thousands to millions of years. Similarly, some rocks return radiometric “ages” twice as old as the accepted age for earth. Most rocks return conflicting radiometric “ages.” In these cases, researchers select results that match what they already believe about earth’s age (see the section Brand New Rocks Give Old “Ages” for details of this study and several others like it).
Overview of Radiometric Dating[ii]
Fossil remains are found in sedimentary rock layers. Layers of sediment form when various size particles (e.g., dirt, rocks, and vegetation) accumulate in places such as deserts, rivers, lakes, and the ocean. Most texts teach that it takes a long time for these sediments to build up, with older layers buried beneath younger layers. Fossils found in lower layers are deemed to be older than those in the upper layers, older on the bottom younger on the top. This is called relative age dating, the first step.
Next, evolutionary scientists then use index fossils to help establish the relative ages of rock layers that are not directly related to one another and their fossils. Index fossils are distinct fossils, usually of an extinct organism found in only one or a few layers, though that layer or layers outcrops in many places—at least that’s the theory. They help establish and correlate the relative ages of rock layers. Index fossils typically have a short stratigraphic or vertical range. In reality, many index fossils occur above or below their expected ranges. In some cases, they turn up still alive today, but these can go unreported. Evolutionists assume that the creature evolved somehow, lived for a certain time period, and then died out. Textbooks are correct when they state that relative dating provides no information whatsoever about a fossil’s absolute age. Nevertheless, most textbook writers and the scientists they rely on grew up with a belief in uniformitarian geologic processes. The principle of uniformity is a philosophy and an assumption that the slow geologic processes going on today must explain the deposits of the past. They teach the motto, “the present is the key to the past.” It’s not. As any judge in court will attest, eyewitness records record the past more accurately. Also, keen observations in the field testify that the sediments comprising the ancient rock layers were laid down catastrophically, not slowly over millions of years.
Today, the geologic time scale shows ages based on radiometric age dating. Many textbook authors consider radiometric ages as absolute ages. However, as you will soon learn, these techniques stray far from absolute dates, though they may reveal relative ages of some rocks.
The Age of the Earth
Today’s evolutionists base their age of the Earth on their interpretation of radioactive elements. They assign 4.5 billion years to earth based on the belief that earth itself evolved, so to speak, from a molten mass. But they cannot directly date the earth using selected isotopes because they believe all rocks have cycled over imagined eons, leaving no original rocks to test. They assume meteorites formed when earth did. Researchers age-dated a meteorite to sometime around the age they would accept. Thus, the earth itself has no direct evidence for its vast evolutionary age assignment.
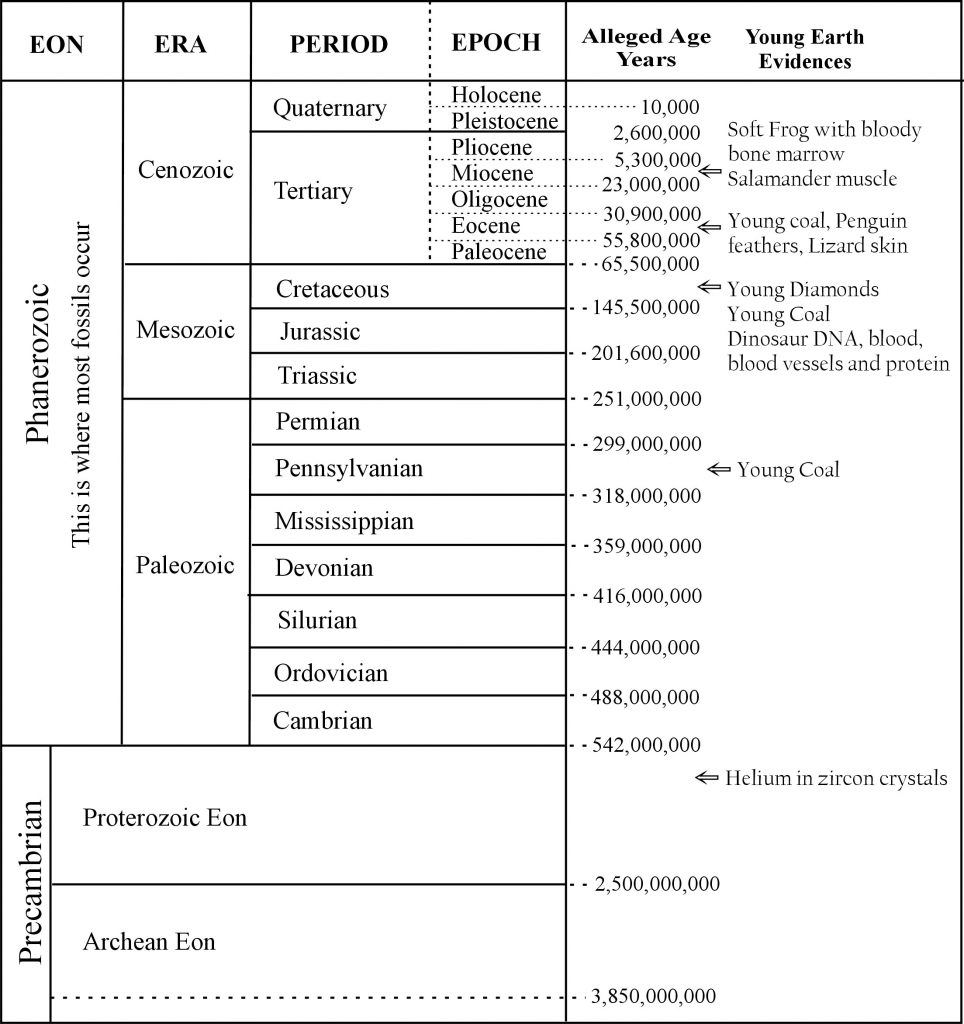
The various rock layers are given names with assigned ages (Figure 1). Those who believe these ever-changing but always unimaginably old age assignments call each rock System a “Period.” The names help, but their age assignments derive from results chosen to agree with evolutionary time. To understand exactly why, we must first learn the basics of radioactive elements and of the techniques used when treating these systems of elements as clocks.
Many elements on the periodic table have radioactive forms. Stable atoms have a set number of protons, neutrons, and orbital electrons. Isotopes are atoms of the same elements with the same number of protons but different numbers of neutrons. Some isotopes are radioactive and others are stable. A radioactive nucleus is not stable. It changes into another element by emitting particles and/or radiation.
A basic way to express the rate of radioactive decay is called the half-life. This equals the length of time needed for 50% of a quantity of radioactive material to decay. Unstable radioactive isotopes called parent elements become stable elements called daughter elements. Each radioactive element has its own specific half-life (see Table 1).
Table 1: Radiometric Isotopes and Half-Lives.
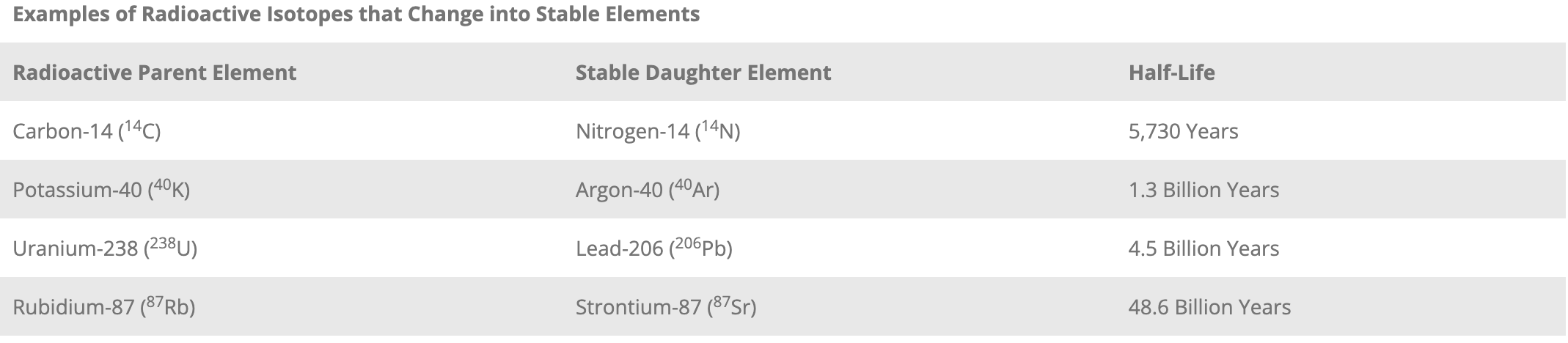
Note: Carbon-14 is not used to date minerals or rocks, but is used for organic remains that contain carbon, such as wood, bone, or shells.
To estimate a radioisotope age of a crystalline rock, geologists measure the ratio between radioactive parent and stable daughter products in the rock. They can even isolate isotopes from specific, crystallized minerals within a rock. They then use a model to convert the measured ratio into an age estimate. The models incorporate key assumptions, like the ratio of parent to daughter isotopes in the originally formed rock. How can anyone know this information? We can’t. We must assume some starting condition. Evolutionists assume that as soon as a crystalline rock cooled from melt, it inherited no daughter product from the melt. This way, they can have their clock start at zero. However, when they find isotope ratios that contradict other measurements or evolution, they often invoke inherited daughter product. This saves the desired age assignments.
Igneous (crystalline) rocks—those that have formed from molten magma or lava—are the primary rock types analyzed to determine radiometric ages. For example, let’s assume that when an igneous rock solidified, a certain mineral in it contained 1,000 atoms of radioactive potassium (40K) and zero atoms of argon (40Ar). After one half-life of 1.3 billion years, the rock would contain 500 40K and 500 40Ar atoms, since 50% has decayed. This is a 500:500 or 500-parent:500-daughter ratio, which reduces to a 1:1 ratio. If the sample contained this ratio, then the rock would be declared 1.3 billion years old. If the ratio is greater than 1:1, then not even one half-life has expired, so the rock would be younger. However, if the ratio is less than 1:1, then the rock is considered older than the half-life for that system.
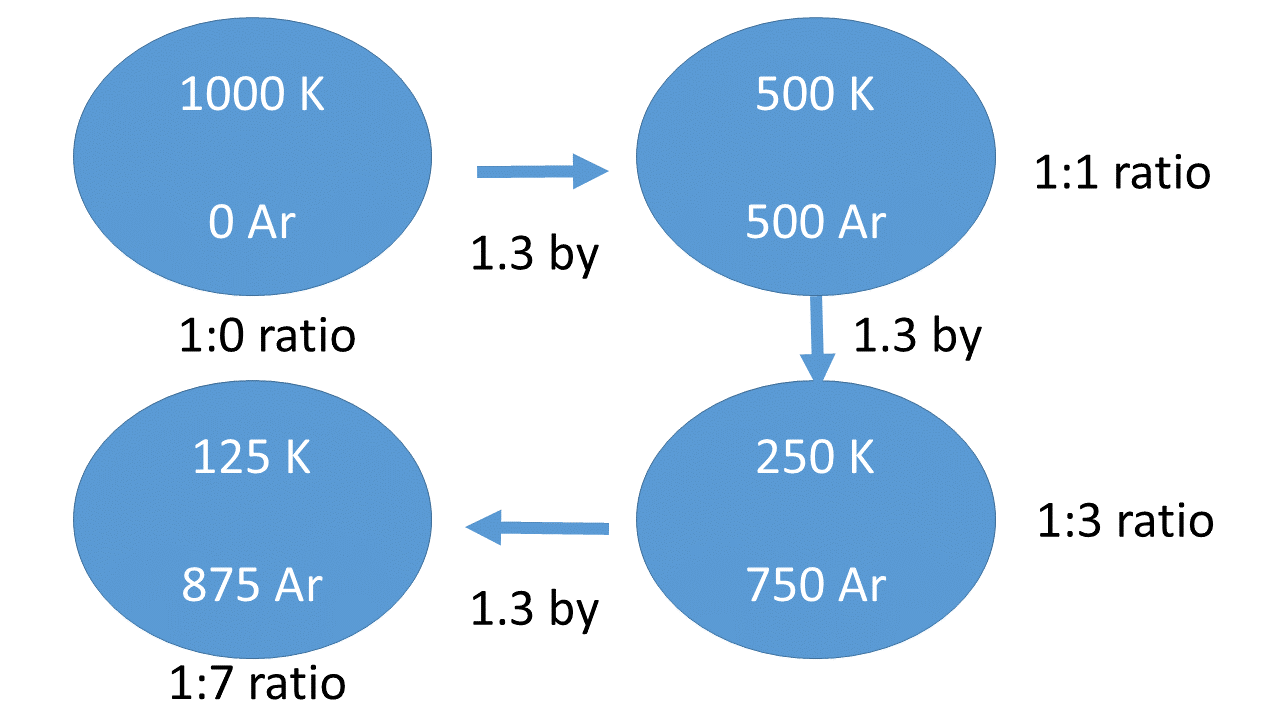
Age-dating a rock requires at least these four basic assumptions:
Assumption #1: Laboratory measurements that have no human error or misjudgments. Measuring the radioactive parent and stable daughter elements to obtain the ratio between them must be accurate, and it usually is. Keep in mind that most laboratory technicians believe in deep time. This sets the time periods they expect. They all memorized the geologic time scale long before they approached their research, and thus may not even consider that processes other than radioisotope decay may have produced the accurately measured isotope ratios.
Assumption #2: The rock began with zero daughter element isotopes. Next, this technician assumes that all the radioactive parent isotopes began decaying right when the mineral crystallized from a melt. He also assumes none of the stable daughter element was present at this time. How can anyone really know the mineral began with 100% radioactive parent and 0% daughter elements? What if some stable daughter element was already present when the rock formed? After all, these experts often explain away unexpected radioisotope age results using the excuse that daughter or parent isotopes must have been present when the rock formed. Without knowledge of the starting condition, the use of isotopes as clocks means nothing.
Assumption #3: The rock maintained a “closed system.” A closed system means that no extra parent or daughter elements have been added or removed throughout the history of the rock. Have you ever seen an atom? Of course not. It is too small, but we must think about this on an atomic level. Decay byproducts like argon and helium are both gases. Neither gas tends to attach to any other atom, meaning they rarely do chemistry. Instead of reacting with atoms in rock crystals, they build up in rock systems and can move in and out of the rocks. One leading expert in isotope geology states that most minerals do not even form in closed systems. A closed system would retain all the argon that radioactive potassium produces. He emphasizes that for a radioactive-determined date to be true, the mineral must be in a closed system.[iii] Is there any such thing as a closed system when speaking of rocks?
Assumption #4: The decay rate remained constant. The constant-decay rate assumption assumes the decay rate remained the same throughout the history of the rock. Lab experiments have shown that most changes in temperature, pressure, and the chemical environment have very little effect on decay rates. These experiments have led researchers to have great confidence that this is a reasonable assumption, but it may not hold true. Is the following quote an overstatement of known science? “Radioactive transmutations must have gone on at the present rates under all the conditions that have existed on Earth in the geologic past.”[iv] Some scientists have found evidence that zircon crystals endured high levels of radioactive decay in the past, as discussed below. This evidence challenges assumption #4.
To illustrate how much radioisotope dating hinges on assumptions, imagine you encounter a burning candle sitting on a table. How long has that candle been burning? We can calculate the answer if we know the candle’s burn rate history and original length. However, if the original length is not known, or if it cannot be verified that the burning rate has been constant, it is impossible to tell for sure how long the candle was burning. A similar problem occurs with radiometric dating of rocks. Since the initial physical state of the rock is unknowable, workers must assume it.”[v]
Brand New Rocks Give Old “Ages”
Scientific literature omitted from public school textbooks reveal radioisotope age assignments much older than the known ages of many rocks. These results first arrived in the 1960s and 1970s, but most of the scientific community still pays no attention. Argon and helium isotopes were measured from recent basalt lava erupted on the deep ocean floor from the Kilauea volcano in Hawaii. Researchers calculated up to 22,000,000 years for brand new rocks![vi] The problem is common. Table 2 gives six examples among many more.
Table 2: Young Volcanic Rocks with Really Old Whole-Rock K-Ar Model Ages.[vii]

The oldest real age of these recent volcanic rocks is less than 500 years. People witnessed and described the molten lava solidify into most of these rocks just decades ago. Many of these were only about 10 years old. And yet 40K-40Ar dating gives ages from 350,000 to >22,800,000 years.
Potassium-Argon (40K-40Ar) has been the most widespread method of radioactive age-dating for the Phanerozoic rocks, where most fossils occur. The misdated rocks shown above violate the initial condition assumption of no radiogenic argon (40Ar) present when the igneous rock formed. There is too much 40Ar present in recent lava flows. Thus, the method gives excessively old ages for recent rocks. The amounts of argon in these rocks indicate they carry isotope “ages” much, much older than their known ages. Could the argon they measured have come from a source other than radioactive potassium decay? If so, then geologists have been trusting a faulty method. If they can’t obtain correct values for rocks of known ages, then why should we trust the values they obtain for rocks of unknown ages?
These wrong radioisotope ages violate the initial condition assumption of zero (0%) parent argon present when the rock formed. Furthermore, the slow radioactive decay of 40K shows that there was insufficient time since cooling for measurable amounts of 40Ar to have accumulated in the rock. Therefore, radiogenic argon (40Ar) was already present in the rocks as they formed.
Radiometric age dating should no longer be sold to the public as providing reliable, absolute ages. Excess argon invalidates the initial condition assumption for potassium dating, and excess helium invalidates the closed-system assumption for uranium dating. The ages shown on the uniformitarian geologic time scale should be removed.
“Young” Fossils in “Old” Mud
Researchers have scoured the Ono Formation near Redding in northern California. They described it in scientific publications for more than 140 years. Because the area has millions of fossils (including the valuable ammonites) and fossilized wood trapped in the same mudflow layers, it provides a unique opportunity for carbon dating. If the wood still has relatively short-lived radiocarbon inside it, then the age of the supposedly ancient fossils would need revision.
Geologist Andrew Snelling gathered four samples of ammonites and wood buried and fossilized together in this solidified mudstone and sent them to the IsoTrace Radiocarbon Laboratory at the University of Toronto, Canada for dating analysis.[viii] Table 3 summarizes the results.
Table 3. Ono Formation Radiocarbon Dating Results.
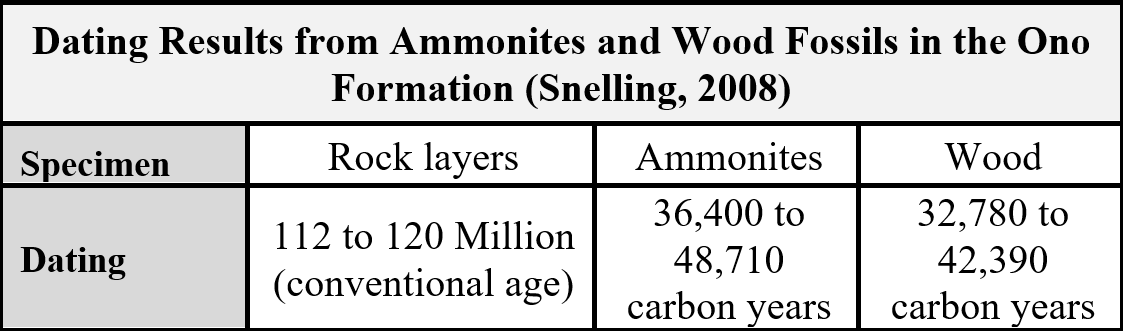
Because the ammonites and wood fossils came from a rock unit conventionally regarded as 112 to 120 million years old, the fossils should share that same age. Such an age far exceeds the limit of the radioactive carbon (14C) method, which in theory extends to artifacts less than 100,000 carbon years old. In other words, if these fossils are really over 100 million years old, then there should have been absolutely no measurable 14C in them—but there was—enough to produce easily measurable ages of 32,000 to 48,000 years!
Scientists who believe in long ages assert that the ammonites and wood samples were contaminated with modern carbon in the ground, during sampling, or even in the laboratory. But this study took extensive steps to guard against such contamination. So how can 36,000 carbon-year-old ammonites and 32,000 carbon-year-old wood be stuck in a mudflow of 112 million or more conventional years? Two logical options present themselves:
Option 1: One of the three dates is correct and the other two are wrong.
Option 2: All three of the dates are wrong.
If Biblical history is accurate as we believe it is, then the second option is the correct choice—none of the dates are correct. The fact that measurable 14C existed in the ammonites and wood fossils shows that they are very young–certainly not 112–120 million years old. But how can they still outdate the Biblical age of Creation of about 6,000 years? A number of factors help explain this. First, the Earth’s stronger magnetic field in the recent past would have reduced the atmospheric 14C production rate. Second, “because the recent Genesis Flood removed so much carbon from the biosphere and buried it, the measured apparent radiocarbon ages are still much higher than the true ages of the fossil ammonites and wood.”[ix]
Therefore, the true ages of the ammonites and wood are consistent with their burial during the Genesis Flood about 4,400 years ago.[x] Back then, muddy waters washed sediments and ammonites onto land.

[i] Kenneth R. Miller and Joseph S. Levine, Biology. (Boston, MA.: Pearson, 2006), p. 466.
[ii] This section was written by Roger Sigler and was carried over from: Daniel A. Biddle (editor), Creation V. Evolution: What They Won’t Tell You in Biology Class (Xulon Press). Roger Sigler, M.S. is a licensed professional geoscientist in the State of Texas and has taught and published in the field of Biblical Creation since 1989.
[iii] Gunter Faure, Principles of Isotope Geology, 2nd ed. (John Wiley & Sons, 1986), 41, 119, 288.
[iv] A.O.Woodford, Historical Geology. (W.H. Freeman and Company, 1965): 191–220.
[v] Judah Etinger, Foolish Faith. (Green Forest, AR.: Master Books, 2003): Chapter 3.
[vi] C.S. Noble and J.J. Naughton, Science, 162 (1968): 265–266.
[vii] Data compiled and modified after Snelling (1998): Andrew Snelling, “The Cause of Anomalous Potassium-Argon ‘Ages’ for Recent Andesite Flows at Mt. Ngauruhoe, New Zealand, and the Implications for Potassium-argon Dating,” in Robert E. Walsh (ed.), Proceedings of the Fourth International Conference on Creationism (1998), p. 503–525. See also: Andrew A Snelling, “Excess Argon”: The “Archilles’ Heel” of Potassium-Argon and Argon-Argon “Dating” of Volcanic Rocks. www.icr.org/article/excess-argon-achillies-heel-potassium-argon-dating/ (February 3, 2016); Steve Austin, “Excess argon within mineral concentrates from the new dacite lava dome at Mount St Helens volcano,” J. Creation 10 (3) (1996): 335–343 (see: www.creation.com/lavadome). (February 3, 2016).
[viii] Andrew Snelling, “Radiocarbon Ages for Fossil Ammonites and Wood in Cretaceous Strata near Redding, California.” Answers Research Journal. 2008 1: 123-144. www.answersingenesis.org/geology/carbon-14/radiocarbon-ages-fossils-cretaceous-strata-redding-california/. (February 3, 2016).
[ix] Ibid.
[x] See earlier endnote regarding biblical genealogies and dating Creation and the Flood.


![[eBook] Thunderbirds and Flying Dragons: Giant Birds & Pterosaurs that Lived After the Flood](https://genesisapologetics.com/wp-content/uploads/2025/12/THUNDERBIRDS-PRODUCT-IMAGE-100x100.jpg)





